.jpg)
September 3, 2025
Strains selected for their antagonism against vaginal and urinary tract pathogens
Bacterial vaginosis (BV), mainly caused by Gardnerella vaginalis, affects 23 to 30% of women globally and costs 5 billion USD annually (1,2) – without taking into account the costs of associated preterm birth and HIV, which triple these costs. Indeed, BV is thought to cause miscarriage, premature birth, pelvic inflammation, and infections including sexually transmitted diseases such as HIV (3). Vulvovaginal candidiasis affects 138 million women globally (above 3%) and costs about 15 billion USD annually from loss of productivity (4).
In their lifetime, 50 to 60% of women experience at least one episode of urinary tract infections (UTIs) (5). UTIs represent 150 million cases each year, for an estimated global healthcare cost of 6 billion USD (6).
A healthy vaginal microbiota, associated with reduced risks of vaginal infections and pregnancy complications such as preterm birth, is characterized by a low-diversity, Lactobacilli-dominated ecosystem that produces anti-pathogen substances, maintains a protective and physiological acidic pH, and stimulates the immune system and mucus secretion (7).
Among the five clusters of vaginal microbiome, types I and II, respectively dominated by L. crispatus and L. gasseri, are associated with healthy outcomes, while cluster IV, which is not dominated by Lactobacilli is considered dysbiotic (8).
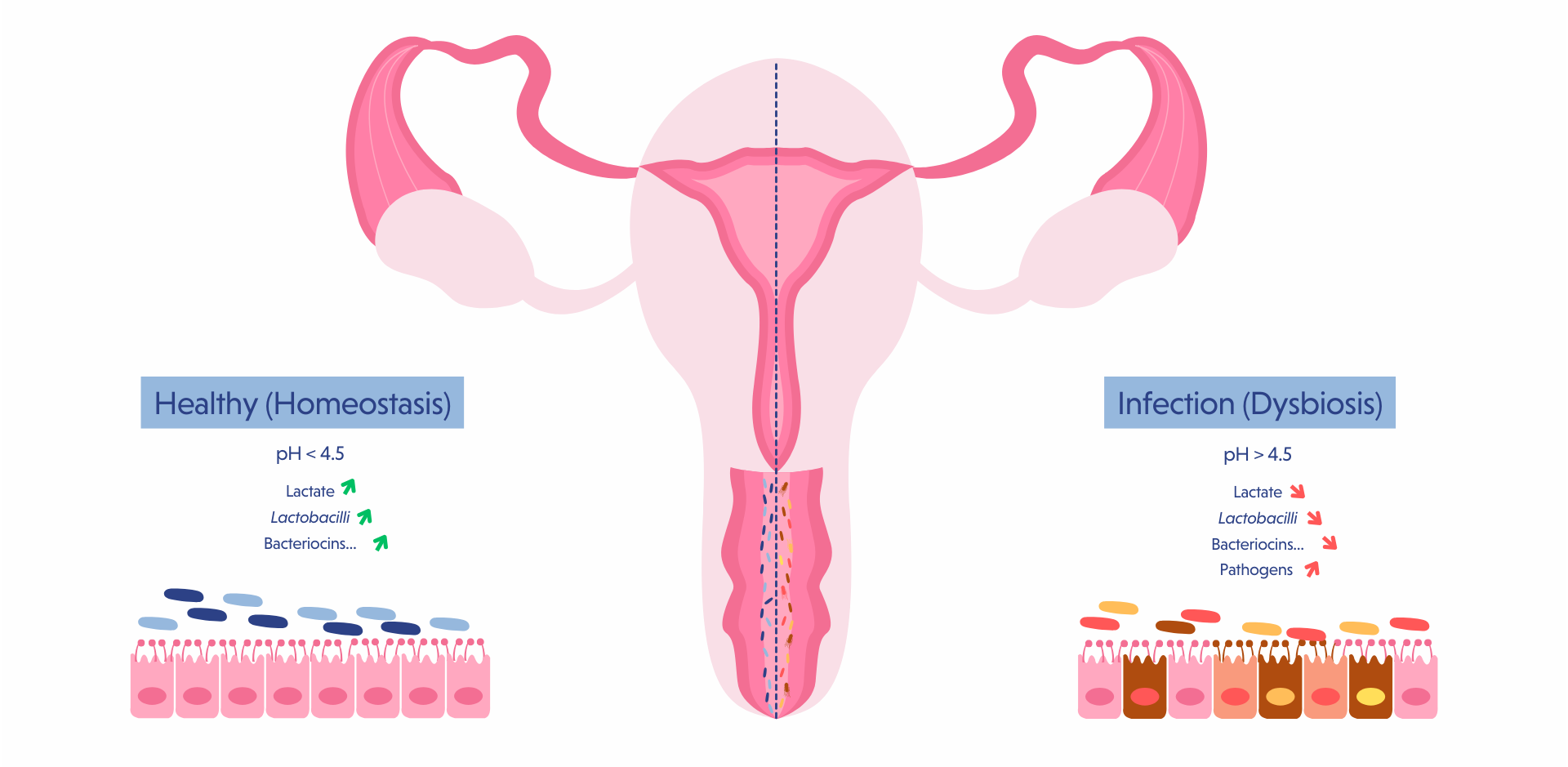
Probiotics consumed orally have been shown to translocate from the gut to the vagina (9,10). In the case of UTIs, the entry of gut bacteria into the urinary tract is facilitated by the short urethra and its proximity to the rectum, which explains why UTIs are more prevalent in women than in men (9).
We selected the strains L. crispatus BIO6272, L. gasseri BIO6369, L. plantarum BIO1096, and L. rhamnosus BIO5326 based on their ability to inhibit major vaginal and urinary tract pathogens and based on literature regarding the protective effects of vaginal ecosystems dominated by L. crispatus and L. gasseri (7,8).
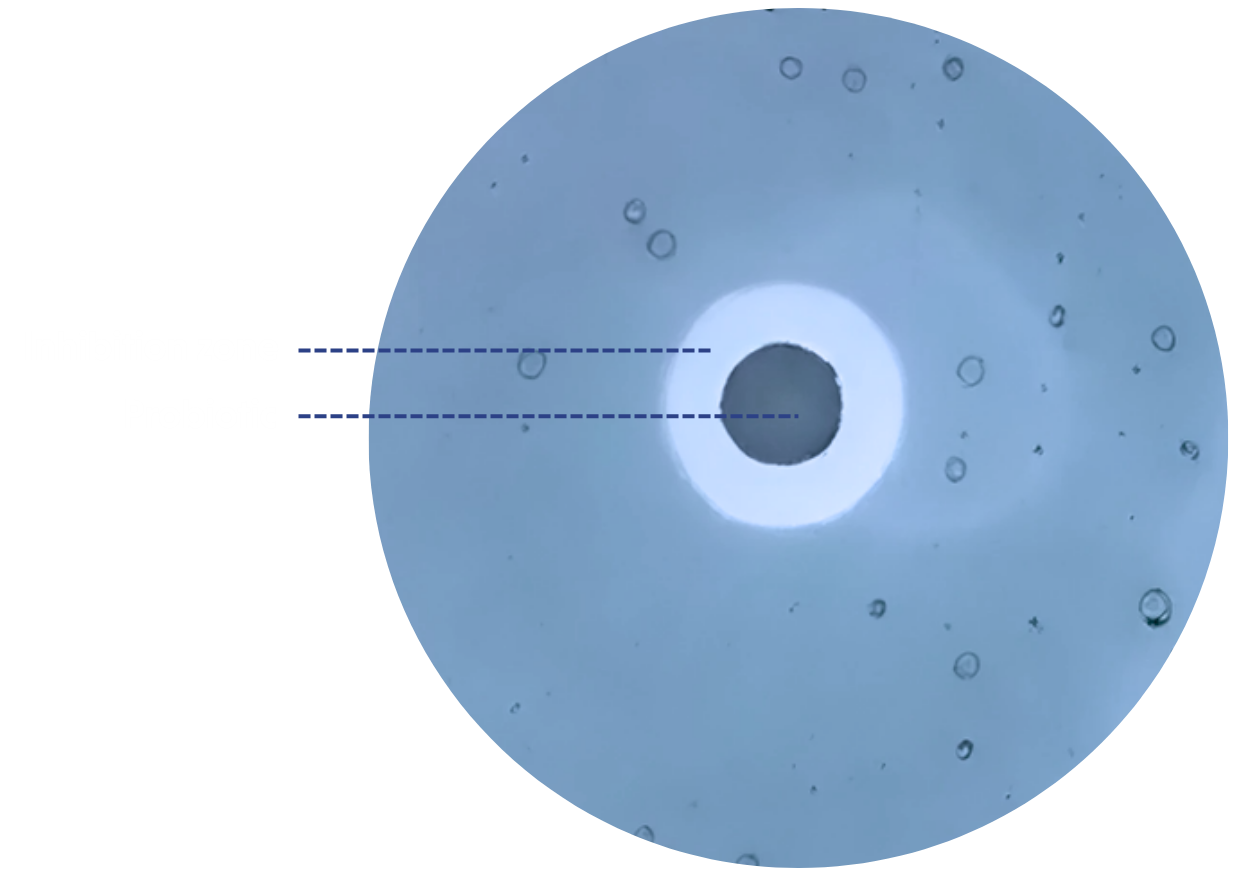
The assessment of pathogens’ inhibition by our probiotics were performed using the agar spot antimicrobial assay. Pathogens were poured over the agar plates containing a spot of probiotics, and the inhibition of pathogen growth was considered positive when a clear zone around the spot was wider than 9 mm.
.png)
The pathogens E. coli, K. pneumoniae, E. faecalis and P. aeruginosa together cause about 90% of all complicated and uncomplicated UTIs (11). F. nucleatum is a pathobiont from the mouth associated with endometriosis (12). Endometriosis affects 10% of women of reproductive age globally (13,14).
.png)
The Agar spot antimicrobial assays highlighted the ability of Bioprox strains to inhibit G. vaginalis as well as the 4 major urinary pathogens.
One of the mechanisms of action of beneficial bacteria in antagonizing vaginal pathogens is mediated by competition for a locus of adhesion (15). Postbiotics (probiotics that were killed by heat treatment), show promise in this context as they can bind to the vaginal epithelium and reduce the ability of pathogens to do the same and multiply.
The method used to evaluate this effect was to incubate VK2/E6E7 vaginal epithelial cells with our postbiotics, followed by the incubation with the vaginal pathogens. After washing away the unbound pathogens, vaginal cells were treated to release the attached pathogens which were then plated for counting. The reduction in adhesion was calculated using a control without postbiotics.
Bioprox strains L. plantarum BIO1096, L. crispatus BIO6272 and L. gasseri BIO6369 in postbiotic form (heat-killed) have been shown to significantly reduce the adhesion of Candida albicans and/or Gardnerella vaginalis on vaginal epithelial cells.
.png)
Bibliography
Interested by our services?